How does the brain store—and access—our memories? While our understanding of the brain has (thankfully) developed greatly from the days of removing cerebral tissue to see what would happen, a precise understanding of where our memories reside—and how our brain recalls past experience—remains elusive.
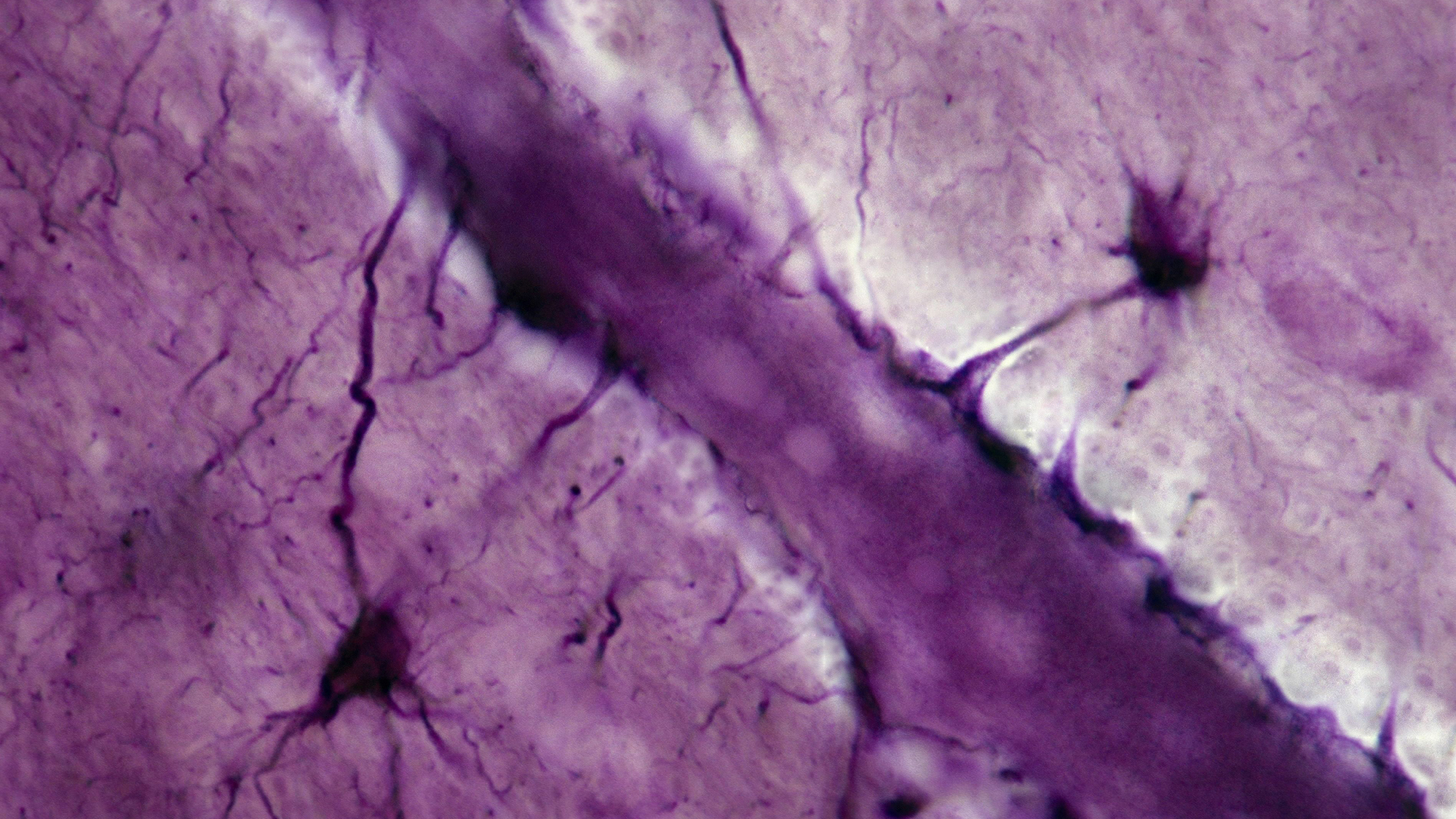
A new paper, published November 6 in Nature, examines the role played by cells called astrocytes—named for their distinctive star-shaped form—in the way that mice process and store memories. The study suggests that these cells are involved in both the formation and storage of memories, and challenges the established theory that memories are stored only in our neurons.
Benjamin Deneen, the paper’s lead author, tells Popular Science that in the early days of neuroscience, research tended to focus on neurons, treating other cells as relatively unimportant support structures. As our understanding of the brain’s intricacies has developed, however, so too has our appreciation of the fact that while our neurons are certainly important, they are not the only cells that contribute to cerebral functionality. In particular, the role of non-neuronal cells called glial cells—of which astrocytes are an example—has undergone wholesale re-examination.
“[Astrocytes] were long viewed as a monolithic support cell in the brain,” says Deneen. However, he continues, “our knowledge of astrocytes has undergone a renaissance over the past 20 years, highlighted by the identification of dynamic physiological activities, key roles in circuit function, and diverse molecular properties.”
The modern understanding is that astrocytes appear to be responsible for a wide variety of functions in the brain—so many, in fact, that it can be difficult to give a simple answer to the question of what they do. “My view is that astrocytes are sort of like the ‘Swiss army knife’ of the brain,” says Deneen. “They interact with practically every cell in the brain, [and] a single astrocyte can contact up to 100,000 synapses.” He cites multiple examples of critical activities for which astrocytes are responsible: “[They] playing an essential role in forming the blood brain barrier; provide metabolic support for neurons, buffer ions and maintaining ion gradients; respond to injury and degeneration; [and] interact with resident immune cells in the brain in response to injury or other insults.”
Nevertheless, despite their importance, we are only beginning to explore the way in which astrocytes participate in the formation and storage of memories.
The prevailing understanding of how our brain stores our memories is that a memory is distributed across the brain in a unique network of neurons called an “engram”. As Deneen explains, “At the cellular level… every memory is comprised of a different ensemble of neurons that are distributed across a host of brain regions–i.e. an engram. Importantly, a single neuron can participate in multiple engrams, but each engram is comprised of a unique ensemble of neurons.”
The study examined how astrocytes in the brains of mice—and, in particular, a specialized variety known as a “learning-associated astrocyte” or LAAs—acted both during and after a learning experience. Researchers found that during such an experience, some of these LAAs were activated; re-activating the LAAs in a different environment appeared to stimulate recall of the memory and associated learning.
Researchers also found that the LAAs activated by a learning experience maintained a high level of a protein expressed by a gene called NFIA. Preventing the production of this protein prevented the recall of the memory of the learning experience in question.
On this evidence, then, it appears that astrocytes play a role in both storage and recall of memories. How does this square with the generally accepted theory that memories are stored as engrams?
“This is a question we’re struggling with in the lab right now,” laughs Deneen. “One way of thinking about it goes back to the fact that astrocytes ‘listen’ to neurons and respond to meet the demands of a functioning circuit. Under learning conditions, where an ensemble of neurons has formed a memory engram, it’s possible that the neurons need to ‘offload’ aspects of the memory to the astrocytes, where the astrocyte serves as a vault or reservoir for the information stored by a given engram neuron.”
Whatever the case, it’s clear there is a great deal more to learn about the nuances of how memory works—and that the apparent involvement of astrocytes both raises many new questions and hints at many new possibilities. “It’s clear that astrocytes are now involved in the recalling of memory,” says Deneen. “Whether they actually store the memory or serve as conduit for retrieval is unknown. Perhaps there is some ‘indexing’ between astrocytes and neurons at the cellular level, where a neuron distributes its memory ‘information’ to a host of LAAs that are in close proximity.”
There are also many more questions to be answered about astrocytes themselves: for example, while LAAs are certainly specialized cells, it’s not straightforward whether there are distinct, predetermined types of astrocyte, or a single cell that evolves to fill whatever roles are required. “I’ve spent over twenty years studying how astrocytes are made and what they do,” says Deneen, “and this is not an easy question to answer… I think there are different subtypes of astrocytes, but it’s not a black-and-white issue. There is evidence that different subtypes of astrocytes are developmentally encoded… but whether this ‘diversity’ reflects a hardwired, preordained state or is an adaptation to their local environment … is still up for debate.”
Source link